Recent Studies in Pancreatic Cancer
Hedgehog Inhibitors for Metastatic Adenocarcinoma of the Pancreas
Phase 2
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University
In this Phase II trial, the purpose is to evaluate the progression free survival in patients with metastatic adenocarcinoma of the pancreas treated with a hedgehog inhibitor (GDC-0449) in combination with chemotherapy (gemcitabine and nab-Paclitaxel). The Hedgehog inhibitor alters intracellular Ca2+ homeostasis and inhibits cell growth in cisplatin-resistant lung cancer cells.
Gemcitabine is a chemotherapy drug used to treat cancer of the bladder, pancreas, ovary and breast, and non-small cell lung cancer. Usually given in the vein. Nab-Paclitaxel is also known as Abraxane . It combines the chemotherapy drug paclitaxel with a protein called albumin.
PFS (progression free survival ) is the length of time during and after the treatment of a disease, such as cancer, that a patient lives with the disease but it does not get worse. In a clinical trial, measuring the progression-free survival is one way to see how well a new treatment works
Description
The emergence of new small molecules with capacity of blocking the Hedgehog signaling pathway provides a novel therapeutic approach in pancreatic adenocarcinoma treating the primary tumor, stroma, systemic metastases and pancreatic cancer stem cells by hedgehog pathway inhibition. This phase 2 clinical trial will evaluate the progression free survival (PFS) in patients with previously untreated metastatic pancreatic adenocarcinoma. We hypothesize that the combination of cytotoxic agents (gemcitabine and nab-paclitaxel) with the Hedgehog inhibitor GDC-0449 may increase PFS.
98 subjects signed consents in total. 26 subjects were screen-fails. Data for this outcome measure was evaluable in only in 67/72 participants.Peripheral blood data was collected from only 57/72 participants.
Pancreatic cancer participants
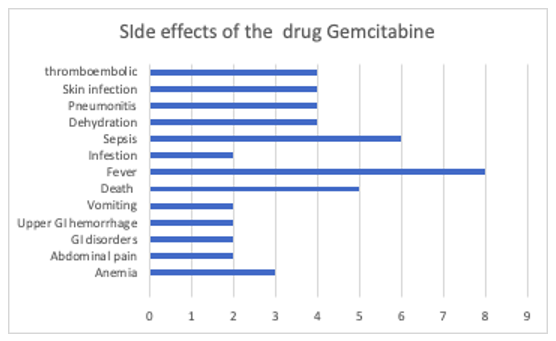
SIDE Affects
Vismodegib and Gemcitabine Hydrochloride in Treating Patients With Advanced Pancreatic Cancer
This pilot clinical trial studies vismodegib and gemcitabine hydrochloride in treating patients with advanced pancreatic cancer. Gemicitibane is a type of chemotherapy that can treat breast, ovarian, non-small cell lung, and pancreatic cancer. It can be used alone or in combination with other medications. Vismodegib may stop the growth of pancreatic cancer by blocking blow flow to the tumor. Gemcitabine hydrochloride may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving vismodegib and gemcitabine hydrochloride may kill more tumor cells.
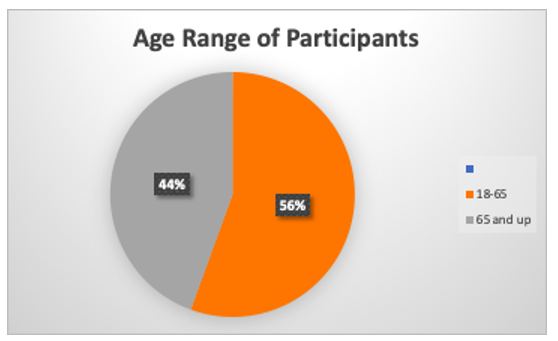
A clinical study involves research on volunteering qualifying participants . For this clinical trial , participants have to be at least 18 years and older. Other criteria for this trial is necessary . Patients must have histologically or cytologically confirmed pancreatic cancer . Patients must have metastatic disease or recurrent disease following surgical therapy. Patients must have measurable disease, defined as at least one lesion that can be accurately measured in at least one dimension. Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study.
In this clinical trial , the main goal is to obtain tumor biopsies before and after therapy with GDC-0449 (vismodegib) to evaluate the effect of inhibition of hedgehog signaling on pancreatic cancer stem cells by: evaluating the tumor for number and percentage of pancreatic cancer stem cells before and after treatment with GDC-0449.
Researchers had 25 participants who were observed and tested within 3 weeks. Within this clinical trial , researchers were looking for the number of participants with either a complete response (CR) or a partial response (PR) will be calculated. A CR is defined as the disappearance of all target lesions. A PR is defined as at least a 30% decrease in the sum of the diameters of target lesions. Each patient received a vismodegib PO QD on days 1-28 and gemcitabine hydrochloride IV over 30 minutes on days 1, 8, and 15 (beginning in course 2). Courses repeat every 28 days in the absence of disease progression or unacceptable toxicity. These are specific interventions according to the research plan or protocol created by the investigators. These interventions may be medical products, such as drugs or devices; procedures; or changes to participants’ behavior, such as diet.
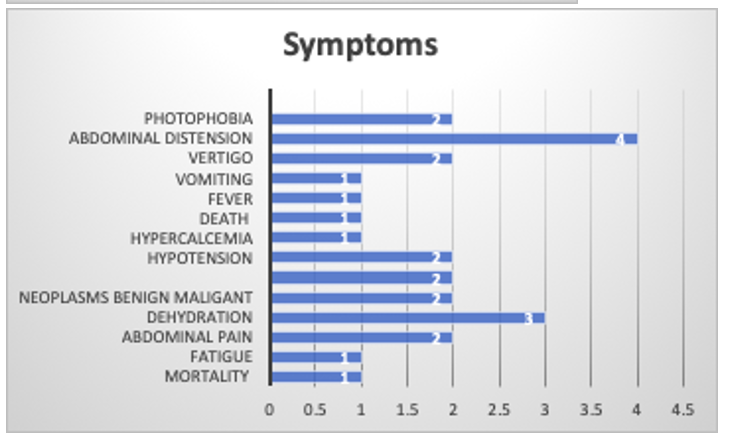
16/25 were successful and dehydration, abdominal pain , nausea , vomiting and constipation was the top side effects
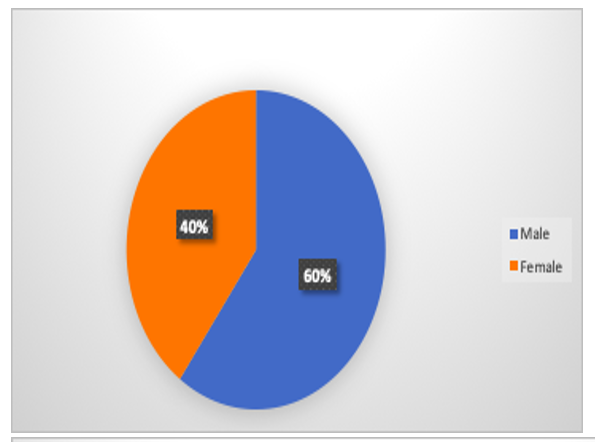
Gender Ratio in Study
Participant gender
Symptoms